The most common error is using Heptane or Methylcyclohexane that is not of the required purity. One theoretical plate is equivalent to one vaporization-condensation cycle which is equivalent to one simple distillation.
The distillation is commonly conducted using a column with 15 theoretical equilibrium stages at a reflux ratio of 5 and is known as the true boiling point TBP distillation.
Theoretical plates fractional distillation. Theoretical Plates Gas chromatography is a powerful means of performing qualitative and quantitative measurements of complex mixtures of volatile substances. The separation process in gas chromatography can be compared to a multiple distillation or a fractional distillation. Let us consider the following situation.
Liquid A with boiling point 80 C. Liquid B with boiling point 150 C. Also assume that A and B are completely miscible and forms an ideal mixture.
Now let us take a mixture containing equal volumes of A and B a 5050 mixture. We want to separat. What are theoretical plates in fractional distillation.
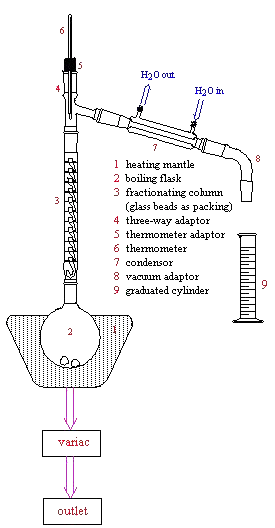
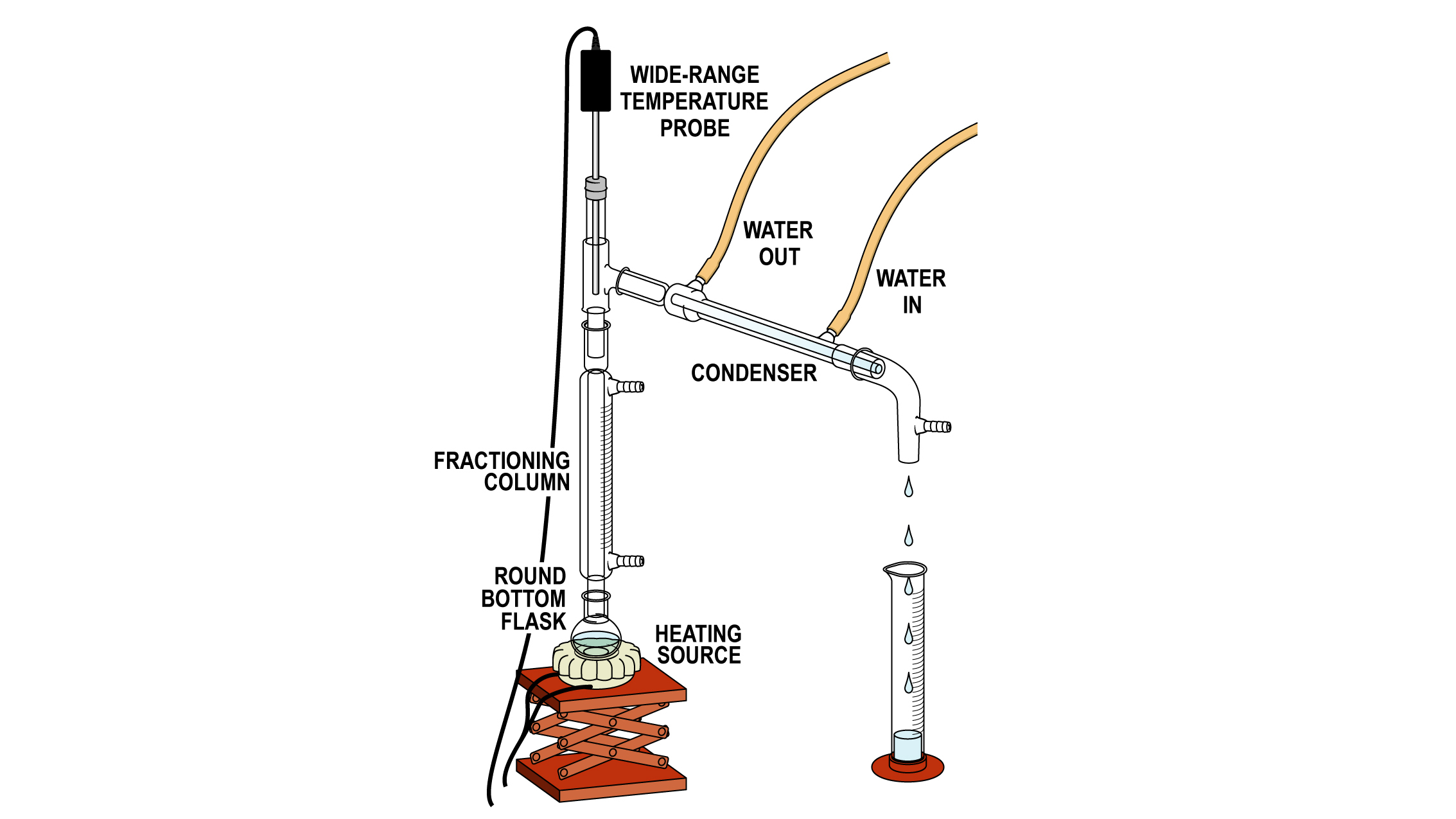
A theoretical plate is an area in the column where the recondensing liquid and the rising vapor come into equilibrium with one another. This applies to a packed column vs. One with actual physical plates or trays.
Theoretical plates and plate height were first applied to fractional distillation columns in the 1930s. It was assumed that a distillation column is composed of a series of contiguous segments or stages each of which contain condensate in equilibrium with. A theoretical plate is simply the distance it takes within the column for one complete vaporization-condensation cycle or simple distillation to occur.
Because vaporization and condensation are equilibrium processes the most important factor in carrying out a good fractional distillation experiment is heating slowly. This allows for equilibrium to be established throughout the column and the liquids. A column is known as the number of theoretical plates of that column.
One theoretical plate is equivalent to one vaporization-condensation cycle which is equivalent to one simple distillation. Thus a fractionation column that can attain the equivalent of three simple distillations would be said to have three theoretical plates. Plural theoretical plates physics chemistry A plate or tray in a distillation column that produces the best possible difference between the liquid and vapour phases in equilibrium with it.
The equivalent concept in a fractionating column packed with beads or rings. The number of theoretical plates NTP is the basic process parameter of every plate column. All the methods that deal with NTP are based on the idea that every plate represents one theoretical.
The more theoretical plates the better the distillation will be. Fractional distillation would have more theoretical plates than simple distillation. The number of theoretical plates is about 032 which is below one plate.
This isnt the best considering that the more plates there are the more distillation will occur. Theoretical plate is a hypothetical zone where evaporation and subsequent condensation occurs. If one performed several simple distillations the liquid would get increasing pure with each distillation however it would take substantial time an effort to perform multiple simple distillations.
A more convenient alternative is a fractional. Fractional distillation Chromatography lab questions 1 Explain Theoretical plates HETP 2Difference between simple and fractional distillation I 3 Explain mode of separation 4 What are the limitations of gas chromatography with TCD. 5What are the potential applications of fractional distillation and gas chromatography in organic chemistry.
Simple vs Fractional Distillation 1 Fractional Distillation. Fractional Distillation is more efficient than the Simple Distillation. If there are an adequate number of theoretical plates the mixture will distill one component at a time in fractions.
With enough plates then each fraction consists of only a single pure substance. ASTM D1160 Vacuum Distillation. Theoretical Plate Study 36-100.
Column is known as the number of theoretical plates of that column. One theoretical plateis equivalent to one vaporization-condensation cycle which is equivalent to one simple distillation. Thus a fractionation column that can attain the equivalent of three simple distillations would be said to have three theoretical plates.
The number of THEORETICAL PLATES are then calculated from the FENSKE EQUATION below. A for the MethylcyclohexaneHeptane mixture is 10760. It is easy to make critical mistakes when performing a plate study.
The most common error is using Heptane or Methylcyclohexane that is not of the required purity. The liquid phase is generally characterised by fractional distillation and measuring the properties of the collected fractions. The distillation is commonly conducted using a column with 15 theoretical equilibrium stages at a reflux ratio of 5 and is known as the true boiling point TBP distillation.
The main fractions collected can then be fractionated a second time if necessary. The experiment we have just discussed is called a simple distillation. It is an experiment that involves a single equilibration between the liquid and vapor.
This distillation is referred to as involving one theoretical plate. As the reflux ratio increases the number of theoretical plates required decreases. The Optimum Reflux Ratio R 0 is that at which the total cost of the distillation is a minimum taking into account the capital cost of the column which depends on the number of theoretical plates and running cost which depends on the reflux ratio.
Note that the capital costs of the reboiler and condenser also depend on the reflux.