Compounds are chemically combined atoms moleculesrepresent the smallest bits of compounds. Compounds contain atoms of two or more elements.

Molecules are atoms of more than two elements that are chemically bonded together.
Relationship among atoms elements and compounds. The sub-atomic particles that represent the individual components of an atom no longer possess the same properties as the element from which they came. Compounds are substances that are created when two or more elements are chemically joined. The atoms of those elements attach to each other by chemical bonds.
Compounds are formed because elements are seldom found in a pure statethey often bond as valence electrons go to other elements in order to provide stability for the atom. Atoms combine to form elements or in some places like in monoatomic elements atoms themselves are elements. Atoms combine with each other of different elements to form compounds.
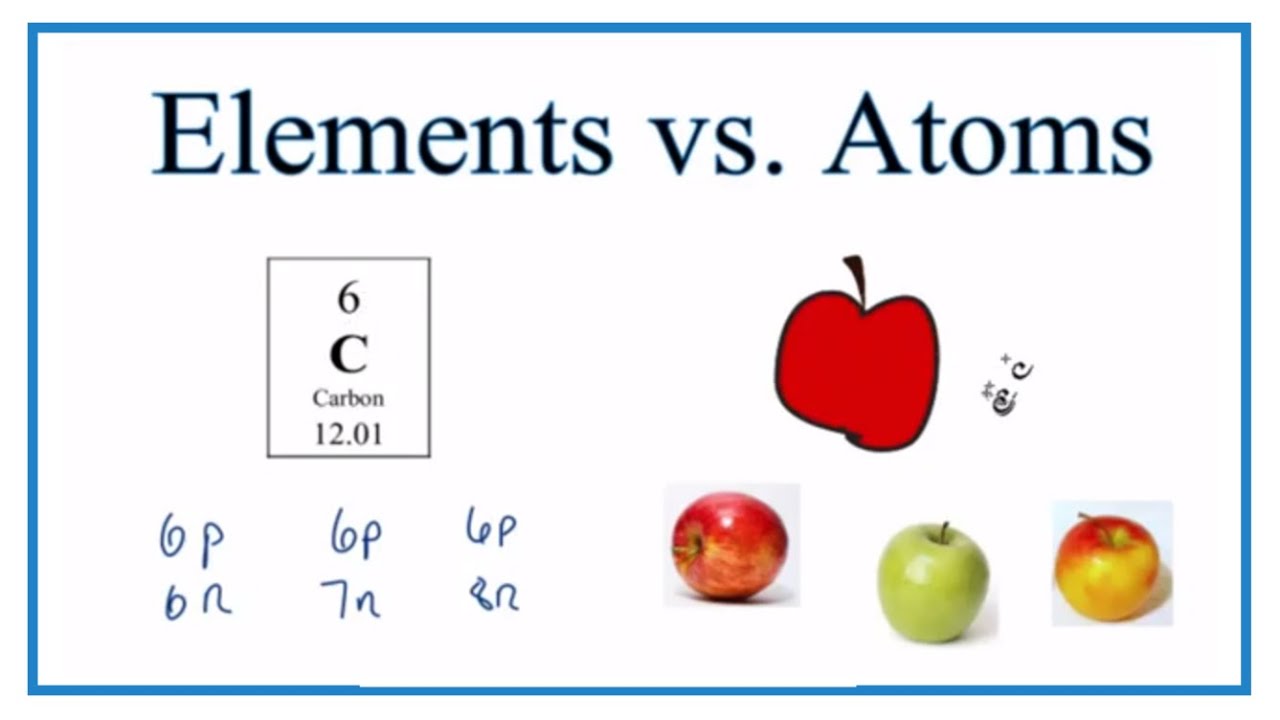
Naturally occurring metallic comppunds in earths crust along with impurities like sand mud certain inorganic compounds like iron oxidetitanium oxide etc. Elements are composed of atoms all of the same kind. The element carbon is made only of carbon atoms.
Compounds are composed of two or more different kinds of atoms chemically joined. Molecules are made of two or more atoms chemically joined by sharing their outer electrons. Atoms of elements combine to form molecules which can be molecular compounds and atoms of elements can combine to form ionic compounds as well.
Compounds contain atoms of two or more elements. Both are pure substances but atoms represent the smallest bitsof elements. Compounds are chemically combined atoms moleculesrepresent the smallest bits of compounds.
All substances are made of atoms. An atom is the smallest part of an element that can exist. Compounds are formed from elements by chemical reactions.
Compounds contain two or more elements chemically combined in fixed proportions and can be represented by formulae using the symbols of the atoms from which they were formed. Your shoes are matter your coffee is matter and so on. Matter is made up of atoms and molecules.
Molecules are atoms of more than two elements that are chemically bonded together. Each atom is a certain element that becomes a compoundmolecule when bonded with other atoms. Explain the relationship among atoms elements and compounds.
Atoms make up different elements which is based on the number of atoms in one element and multiple elements join to create a compound. How are atoms and compounds held together. A molecule is formed when two or more atoms from the same element are chemically bonded whereas a compound is defined as the chemical bonding between atoms of different elements.
It consists of identical atoms of gold and all the atoms have the same properties. When two or more elements combine in a certain fixed ratio they form a compound. Atoms and molecules follow the rules of chemistry and physics even when theyre part of a complex living breathing being.
If you learned in chemistry that some atoms tend to gain or lose electrons or form bonds with each other those facts remain true even when the atoms or molecules are part of a. They are all natures of matter atoms make elements and elements make compounds. Furthermore elements and compounds are pure substances that exist in nature.
Most noteworthy the main difference between element and compound is that the former comprises of same types of atoms while the later comprises of different elements. Definition of Element An element refers to a substance that comprises only one type of atom. An element is the simplest type of substance.
One that is made up of identical atoms such as hydrogen. Once a different type of atom is combined with the atoms in an element a compound is created. Like when oxygen is combined with hydrogen and water is formed.
Chemical elements are the basic components of all matter in the universe at the atomic level or larger. An element refers to a specific type of atom. An element is matter made of only one type of atom.
A compound is a substance that contains two or more elements. A heterogeneous mixture is not a solution because the substances that make up a heterogeneous mixture are not evenly mixed. The substances that make up a solution or a homogeneous mixture are evenly mixed.
Every compound molecule or element you come across will be made of atoms. For example humans are made of atoms. Air is made of atoms.
Your computer made of atoms. Everything is made of atoms. This is why you cant begin to understand the difference between atoms and elements without first understanding what an atom is and what its.