
H2SO4 KOH H2O K2SO4 Chemical Equation Details. H_2SO_4aq 2KOHaq rarr K_2SO_4aq 2H_2Ol Alternatively.
To balance a chemical equation enter an equation of a chemical reaction and press the Balance button.
H2so4 koh balanced equation. H2SO4 KOH K2SO4 H2O 1. Label Each Compound With a Variable a H 2 SO 4 b KOH c K 2 SO 4 d H 2 O 2. Create a System of Equations One Per Element H.
2 a 1 b 0c 2 d S. 1 a 0b 1 c 0d O. 4 a 1 b 4 c 1 d K.
0a 1 b 2 c 0d 3. H2SO4 KOH H2O K2SO4 Chemical Equation Details. H2SO4 KOH K2SO4 H2O Chemical reaction and equation.
What are the products of the following reaction H2SO4 Koh. Sulfuric acid react with potassium hydroxide to produce potassium sulfate and water. To balance KOH H2SO4 K2SO4 H2O youll need to be sure to count all of atoms on each side of the chemical equation.
Balanced neutralization reaction between h2so4 and koh. Click to see full answer. Furthermore what is the balanced equation for h2so4 and Koh.
Chemical Equation Balancer H2SO4 KOH K2SO4 H2O. Write the balanced chemical equation between H2SO4 and KOH in aqueous solution. By signing up youll get thousands of step-by-step.
A formula for neutralization of H2SO4 by KOH is H2SO4aq 2KOHaq – K2SO4aq 2H2Ol. This reaction between sulfuric acid and potassium hydroxide creates salt and water. The formula H2SO4aq 2KOHaq – K2SO4aq 2H2Ol represents a neutralization reaction of the acidic sulfuric acid and the alkaline potassium hydroxide.
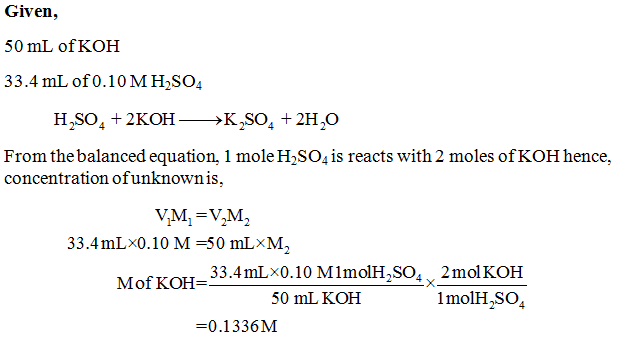
Given chemical equation is. KOH H 2 SO 4 K 2 SO 4 H 2 O Balanced equation is. 2KOH H 2 SO 4 K 2 SO 4 2H 2 O.
To balance this reaction means we need to equalize the number of these above atoms and polyatomic ion from both sides. Step-3Now balance K atom firstIn the left hand sidewe have one K but in the right sidewe have two KSo to balance K we need to put 2 before KOH in the left side. H₂SO₄ 2K OH —–K₂SO₄ H₂O Step-3Now balance H.
H 2 SO 4 2KOH K 2 SO 4 2H 2 O. Sulfuric acid react with potassium hydroxide to produce potassium sulfate and water. How to balance KOHH2SO4K2SO4H2OChemical equation KOHH2SO4K2SO4H2OReaction balance KOHH2SO4.
Write the balanced molecular equation for the neutralization reaction between h2so4 and koh. 2 KOH aq H2SO4 aq K2SO4 aq 2 H2O l This is an acid-base reaction neutralization. KOH is a base H2SO4 is an acid.
What is the complete ionic equation for the reaction 2KOHaqH2SO4aq2H2OlK2SO4aq. Simply so what is the balanced equation for h2so4 and Koh. Chemical Equation Balancer H2SO4 KOH K2SO4 H2O.
What is the product of Koh h2so4. H2SO4 KOH K2SO4 H2O Chemical reaction and equation. 32 Related Question Answers Found.
Examples of the chemical equations reagents a complete equation will be suggested. H 2 SO 4 K 4 FeCN 6 KMnO 4. CaOH 2 H 3 PO 4.
Na 2 S 2 O 3 I 2. C 8 H 18 O 2. Propane oxygen Related chemical tools.
Chemical equations balanced today. Same compounds should not be present in both products and reagents. Equation can be balanced in an infinite number of ways.
This is a combination of two different reactions. Please correct your reaction or click on one of the suggestions below. H2SO4 HCl Cl2 H2O SO2 H2SO4 HCl H3ClSO4 H2SO4 HCl ClS H3O4.
H_2SO_4aq 2KOHaq rarr K_2SO_4aq 2H_2Ol Alternatively. Sulfuric acid potassium hydroxiderarrpotassium sulfate water In the symbol equation both mass and charge are balanced as is mandatory. The balanced chemical equation.
2 KOH H₂SO₄ K₂SO₄ 2 H₂O. Potassium hydroxide will react with sulfuric acid to produce potassium sulfate and water. To balance a chemical equation enter an equation of a chemical reaction and press the Balance button.
The balanced equation will appear above. Use uppercase for the first character in the element and lowercase for the second character. Fe Au Co Br C O N F.
Ionic charges are not yet supported and will be ignored. In this video well balance the equation KOH H2SO4 K2SO4 H2O and provide the correct coefficients for each compound. To balance KOH H2SO4 K2SO4 H2O youll need to be sure to count all of atoms on each side of the chemical equation.
Write the balanced equation for the neutralization reaction between h2so4 and koh in aqueous solution. What is the complete ionic equation for the reaction 2KOHaqH2SO4aq2H2O lK2SO4 aq. One may also ask what is the balanced equation for h2so4 and Koh.
Chemical Equation Balancer H2SO4 KOH K2SO4 H2O. What is the product of Koh h2so4. H2SO4 KOH K2SO4 H2O Chemical reaction and equation.
Write the balanced equation for the neutralization reaction between h2so4 and koh in aqueous solution. Categories Question-Answer Leave a Reply Cancel reply. H2SO4 KOH K2SO4 H2O Chemical reaction and equation.
Is Koh an acid or base. Potassium hydroxide KOH is a strong base as it splits into its atoms and hydroxide ions in water solution. The balanced equation for the reaction of H2SO4 with KOH is as followsH2SO4 2 KOH K2SO4 2H2O A molecular equation is a balanced chemical equation in which the ionic compounds are expressed as molecules rather than component ions.
52886 results page 87 Math 8th Grade.