H 8 FeN 2 O 8 S 2 6 H 2 O. Acts as an iron ion donor for building Fe-S clusters in vitro.
Are you sure I have got the formula mass right.
Ferrous ammonium sulfate hexahydrate molar mass. Ferrous ammonium sulfate hexahydrate is a hydrate that is the hexahydrate form of ferrous ammonium sulfate. Acts as an iron ion donor for building Fe-S clusters in vitro. It is a hydrate and an iron molecular entity.
It contains a ferrous ammonium sulfate anhydrous. Ferrous ammonium sulfate hexahydrate CAS Number. H 20 FeN 2 O 14 S 2.
100 C declit MSDS. Ferrous ammonium sulfate anhydrous is a compound of ammonium iron and sulfate in which the ratio of ammonium to iron 2 to sulfate ions is 212. It has a role as a ferroptosis inducer.
It is a metal sulfate an iron molecular entity and an ammonium salt. It contains an iron 2. Ferrous Ammonium Sulfate Hexahydrate Chemical name.
Iron II Ammonium Sulfate Hexahydrate CAS-No. 7783-85-9 Product code. Recommended use and restrictions on use.
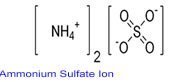
Use of the substancemixture. For laboratory and manufacturing use only. Ferrous Ammonium Sulfate Hexahydrate NH42FeSO42 6H2O bulk research qty manufacturer.
Properties SDS Applications Price. Chromatography and Mass Spectrometry Reagents. Chromatography Columns and Cartridges.
H 8 FeN 2 O 8 S 2 6 H 2 O. Mohr Salt Iron II Ammonium Sulfate Hexahydrate. Ammonium ironII sulfate hexahydrate.
Mohrs salt Ammonium ironII sulfate Ammonium ferrous sulfate hexahydrate Ammonium ironII sulfate hexahydrate. Browse Ammonium ironII sulfate hexahydrate and related products at MilliporeSigma. Product name Ferrous Ammonium Sulfate Hexahydrate Fine Crystals Ammonium Ferrous Sulfate GR ACS Page 2 of 8 SECTION 4.
First aid measures Description of first-aid measures Inhalation After inhalation. Skin contact In case of skin contact. Take off immediately all contaminated clothing.
Rinse skin with water shower. The mass and molarity of chemical compounds can be calculated based on the molar mass of the compound. The mass in grams of a compound is equal to its molarity in moles multiply its molar mass.
Grams mole molar mass. Molar mass of NaCl is 58443 how many grams is 5 mole NaCl. Grams 58443 5 292215 g.
Amonium besi II sulfat heksahidrat MSDS material safety data sheet or SDS CoA and CoQ dossiers brochures and other available documents. 39214 gmol Hill Formula. FeSO 4NH 4 2 SO 46H 2 O hexahydrate Molar mass.
28405 g mol 1 anhydrous 39214 g mol 1 hexahydrate Appearance Blue-green solid Density. 100 to 110 C 212 to 230 F. 373 to 383 K Boiling point.
Molar mass of Fe is 55845g. 01g Fe² 1mol 55845g 179x10³ moles Fe² The Fe² comes from ferrous ammonium sulfate hexahydrate as 1 mole of this salt contains 1 mole of Fe² moles of the salt you need are. The molar mass of the hydrated salt is 39213 gmole.
Hydrated salts include water molecules in their structure that are not chemically bonded to the compound. Ferrous ammonium sulfate CAS number 10045-89-3. 4536 kg Other Regulations.
Hazardous by definition of Hazard Communication Standard 29 CFR 19101200. Ammonium iron II sulfate hexahydrate MSDS material safety data sheet or SDS CoA and CoQ dossiers brochures and other available documents. 39214 gmol Hill Formula.
NH42Fe SO426H2O is a blue green crystal at room temperature. It is soluble in water. Its melting point is 100 C 212 F density 186 gcm3.
Mohrs salt may cause burns to eye and skin. It may cause digestive tract irratation if swallowed. Its widely used such as for water purification or laboratory reagent.
Moles of ferrous ammonium sulfate hexahydrate in Sample 1. The number of moles of anything is calculated from the mass of sample and the molar mass. The molar mass of FeNH 4 2.
Mohr Salt Diammonium Iron II Sulphate 6-Hydrate ferrous ammonium sulphate 6H2O Iron II ammonium sulfate ferrous ammonium sulfate 6hydrate ferrous ammonium sulphate hydrated Iron II ammonium sulfate hexahydrate ferrous ammonium sulfate hexahydrate CAS 10045-89-3 anhydrous. Use the molecular formula to find the molar mass. To obtain the number of moles divide the mass of compound by the molar mass of the compound expressed in grams.
What is FeSO4 nh4 2so46h2o. Mohrs salt or ferrous ammonium sulphate FeSO4 NH4 2 SO4. 6H2O Hydrated ferrous ammonium sulphate is commonly known as mohrs salt.
We simply take the quotient. Fe Mass of iron Mass of hydrated salt 100. 558 g 39213 g mol1.
Are you sure I have got the formula mass right. Post your work if you get stuck. The molar mass of ferrous ammonium sulfate hexahydrate is 3921.
This monograph for Ferrous Ammonium Sulfate Hexahydrate provides in addition to common physical constants a general description including typical appearance applications and aqueous solubility. The monograph also details the following specifications and corresponding tests for verifying that a substance meets ACS Reagent Grade specifications including. Ferrous ammonium sulfate hexahydrate.
Weigh accurately about 007 g of pure ferrous ammonium sulfate hexahydrate dissolve it in water and transfer the solution to a 1-liter volumetric flask. Add 25 mL of concentrated sulfuric acid and dilute the solution to the mark. Calculate the concentration of the solution in.